FULL TITLE:
Gradients of neurotransmitter receptor expression in the macaque cortex
SPECIES:
Macaque
DESCRIPTION:
Data provided:
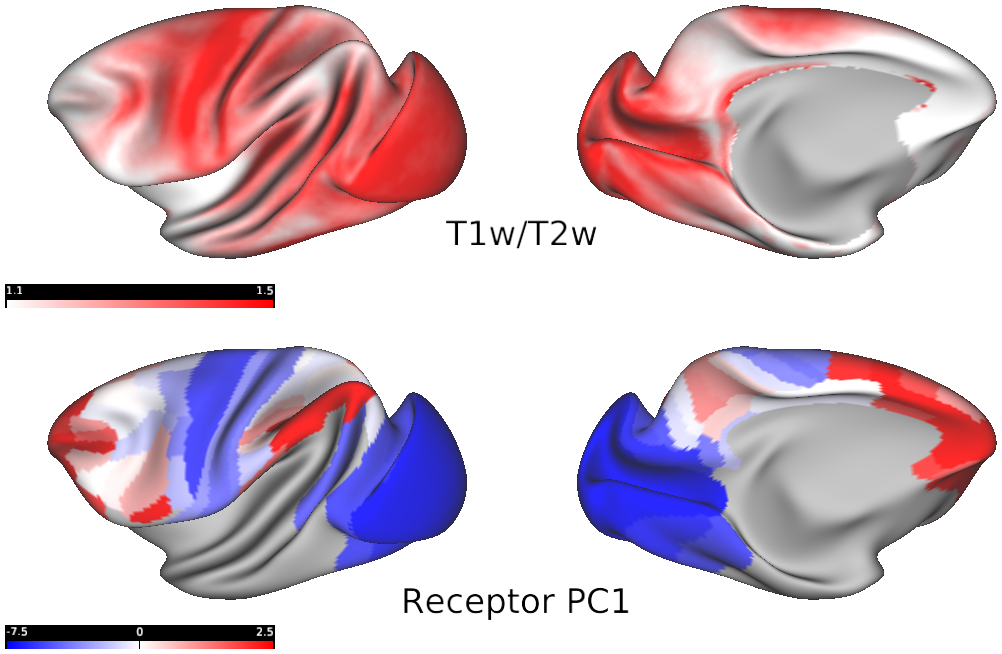
Receptor gradients (this study, Froudist-Walsh et al., 2023).
Receptor data per neuron & raw densities (this study, Froudist-Walsh et al., 2023; Niu et al., 2020; Niu et al., 2021; Rapan et al., 2021; Rapan et al., 2022; Impieri et al., 2019; Froudist-Walsh et al., 2021).
Receptor list:
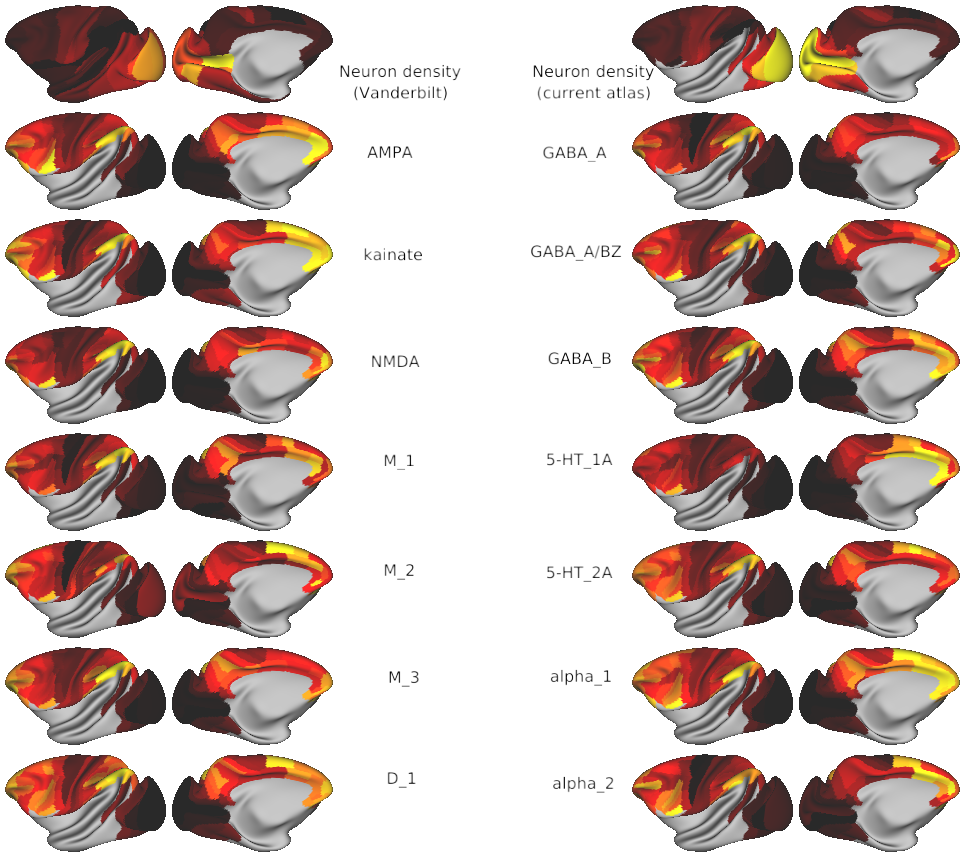
Glutamate: AMPA, kainate, NMDA
GABA: GABA_A, GABA_A/BZ, GABA_B
Acetylcholine: M_1, M_2, M_3
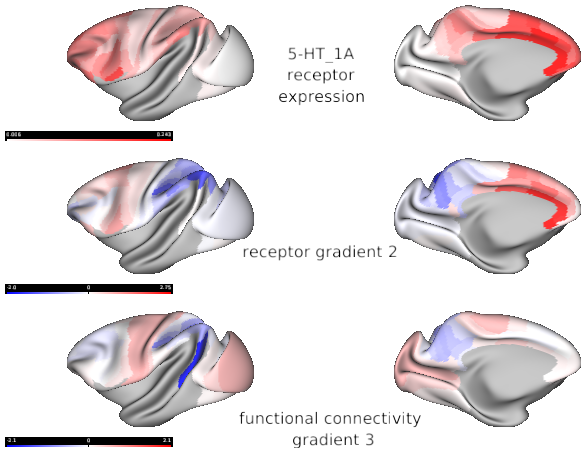
Serotonin 5-HT_1A 5-HT_2A
Noradrenaline/norepinephrine: alpha_1, alpha_2
Dopamine: D_1
Surface representations (in the Yerkes19 cortical space) of the following previously-published data :
Neuron density (source: Collins et al., 2010)
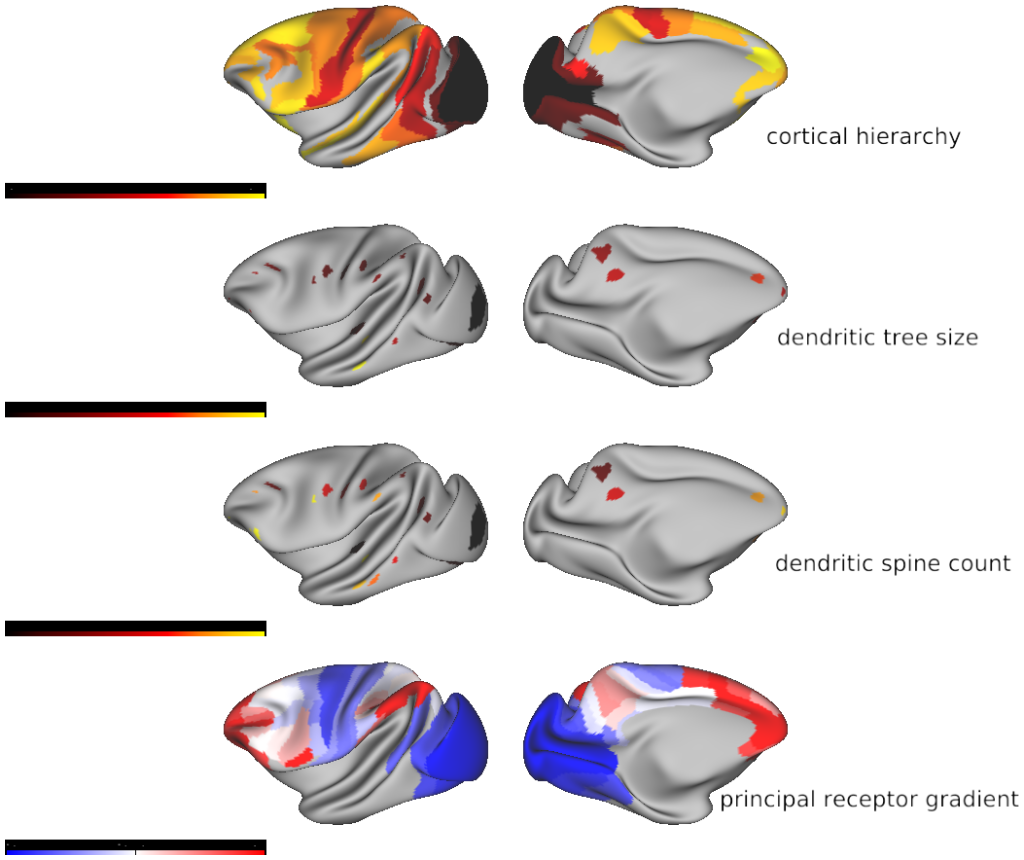
Cortical hierarchy (source: Froudist-Walsh et al., 2021)
Dendritic tree size (source: Elston et al., various, reviewed in Elston, 2007)
Dendritic spines (source: Elston et al., various, reviewed in Elston, 2007)
T1w/T2w map (source: Donahue et al., 2016)
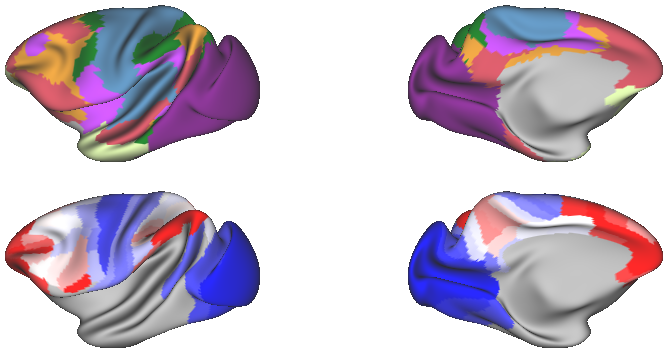
Cognitive networks in macaque brain (source: Xu et al., 2020, Yeo*, Krienen*, et al., 2011)
Macaque functional connectivity gradients (source: Xu et al., 2020)
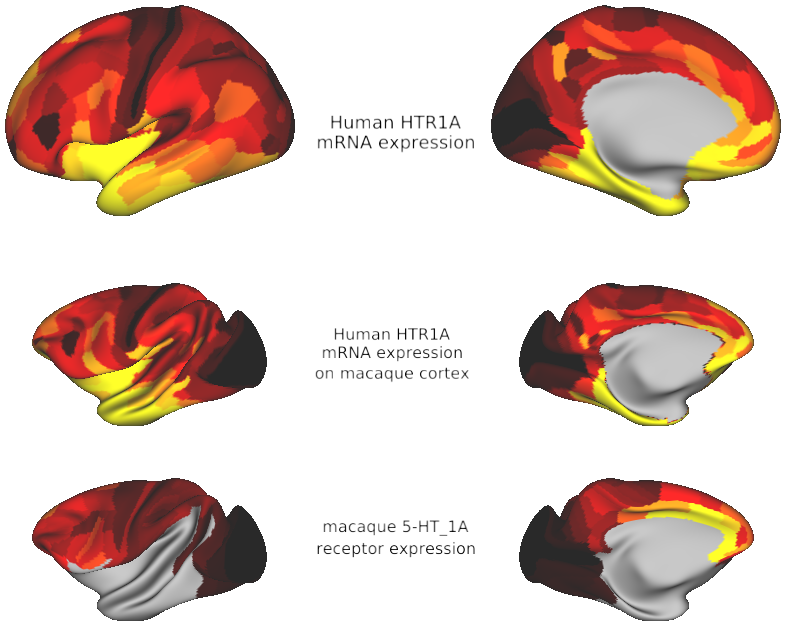
HTR1A gene expression on human & macaque cortex (source: this paper and Hawrylycz et al., 2012)
Code for visualisation and analysis of gene expression: github.com/seanfw/genemapper
Code for processing, visualisation and analysis of other maps: github.com/seanfw/receptorgradients
Data available in table format in MEBRAINS and Lyon (Markov, Kennedy et al., M132) parcellations via EBRAINS at https://search.kg.ebrains.eu/instances/e39a0407-a98a-480e-9c63-4a2225ddfbe4
References:
this paper:
Froudist-Walsh, Sean, Ting Xu, Meiqi Niu, Lucija Rapan, Ling Zhao, Daniel S. Margulies, Karl Zilles, Xiao-Jing Wang, and Nicola Palomero-Gallagher. "Gradients of receptor expression in the macaque cortex." Nature Neuroscience (2023, in press)
Other sources of raw data:
Collins, Christine E., David C. Airey, Nicole A. Young, Duncan B. Leitch, and Jon H. Kaas. "Neuron densities vary across and within cortical areas in primates." Proceedings of the National Academy of Sciences 107, no. 36 (2010): 15927-15932.
Dockès, J. et al. NeuroQuery, comprehensive meta-analysis of human brain mapping. eLife 9, e53385 (2020).
Donahue, C. J. et al. Using diffusion tractography to predict cortical connection strength and distance: a quantitative comparison with tracers in the monkey. J. Neurosci. 36, 6758–6770 (2016).
Elston, G. N. Specialization of the neocortical pyramidal cell during primate evolution. in Evolution of Nervous Systems 191–242 (Elsevier, 2007). doi:10.1016/B0-12-370878-8/00164-6
Froudist-Walsh, S. et al. A dopamine gradient controls access to distributed working memory in the large-scale monkey cortex. Neuron 109, 3500-3520.e13 (2021).
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M; WU-Minn HCP Consortium. (2013) The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80:105-24. (doi: 10.1016/j.neuroimage.2013.04.127) PMID: 23668970
Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012).
Impieri, D. et al. Receptor density pattern confirms and enhances the anatomic-functional features of the macaque superior parietal lobule areas. Brain Structure and Function 224, 2733–2756 (2019).
Niu, M. et al. Receptor-driven, multimodal mapping of cortical areas in the macaque monkey intraparietal sulcus. eLife 9, e55979 (2020).
Niu, M. et al. Organization of the macaque monkey inferior parietal lobule based on multimodal receptor architectonics. NeuroImage 231, 117843 (2021).
Rapan, L. et al. Multimodal 3D atlas of the macaque monkey motor and premotor cortex. NeuroImage 117574 (2021).
Rapan, Lucija, Sean Froudist-Walsh, Meiqi Niu, Ting Xu, Ling Zhao, Thomas Funck, Xiao Jing Wang, Karin Amunts, and Nicola Palomero-Gallagher. "Cytoarchitectonic, receptor distribution and functional connectivity analyses of the macaque frontal lobe." bioRxiv (2022): 2022-06.
Xu, T. et al. Cross-species functional alignment reveals evolutionary hierarchy within the connectome. NeuroImage 223, 117346 (2020).
Yeo, B. T. T., Krienen, F et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology 106, 1125–1165 (2011).
ABSTRACT:
Dynamics and functions of neural circuits depend on interactions mediated by receptors. Therefore, a comprehensive map of receptor organization across cortical regions is needed. Here we use in-vitro receptor autoradiography to measure the density of 14 neurotransmitter receptor types in 109 areas of macaque cortex. We integrate the receptor data with anatomical, genetic and functional-connectivity data into a common cortical space. We uncover a principal gradient of receptor expression per neuron. This aligns with the cortical hierarchy from sensory cortex to higher cognitive areas. A second gradient, driven by serotonin 5-HT1A receptors, peaks in the anterior cingulate, default-mode and salience networks. We find a similar pattern of 5-HT1A expression in the human brain. Thus the macaque may be a promising translational model of serotonergic processing and disorders. The receptor gradients may enable rapid, reliable information processing in sensory cortical areas and slow, flexible integration in higher cognitive areas.
PUBLICATION:
Nature Neuroscience
- DOI:
10.1038/s41593-023-01351-2
- Sean Froudist-Walsh
- Ting Xu
- Meiqi Niu
- Lucija Rapan
- Ling Zhao
- Daniel S. Margulies
- Karl Zilles
- Xiao-Jing Wang
- Nicola Palomero-Gallagher
- New York University
- University of Bristol
- Research Centre Jülich
-
macaque_receptor_gradients.scene
DESCRIPTION: Gradients of neurotransmitter receptor expression in the macaque cortex
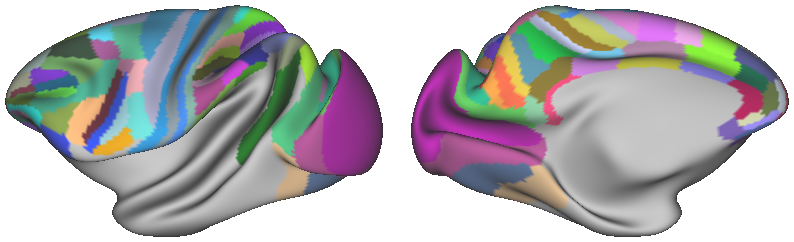
SCENES:- Julich_Macaque_Architectonic_Maps_yerkes19template - Julich Macaque Architectonic Maps
- Fig1_receptors_per_neuron_yerkes19template - The density of 14 receptors per neuron across macaque cortex
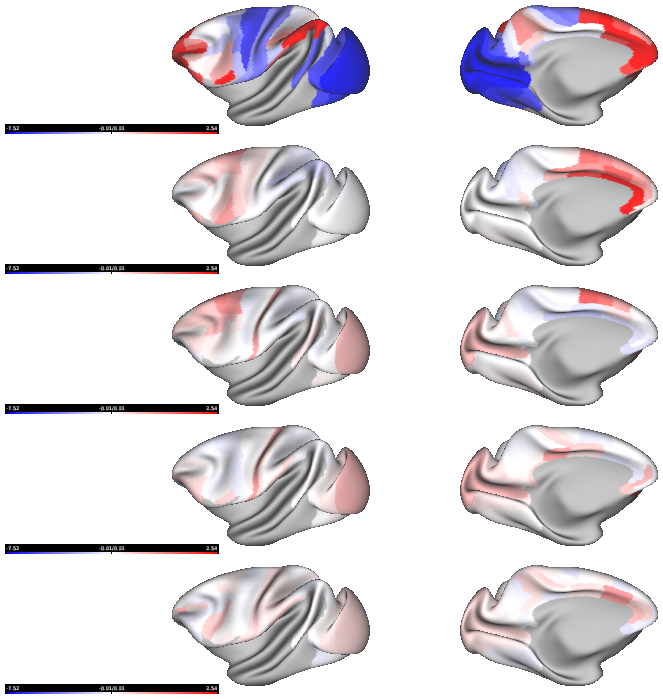
- Fig2_receptor_gradients_per_neuron_yerkes19template - The principal receptor gradient captures total receptor density per neuron across macaque cortex
- Fig3_hierarchy_dendrites_vs_receptor_gradient1 - The anatomical foundations of the principal receptor gradient
- Fig4_myelin_vs_receptor_gradient1 - An inverse relationship between cortical myelin and receptor density
- Fig5_yeo_krienen_functional_networks - The principal receptor gradient separates sensory and cognitive networks
- Fig6_receptor_gradient2
- Fig7_5HT1A_across_species - Serotonin 5-HT1A receptor expression across the human and macaque cortex